[h=1]Water Loss, Acid Stratification and Surface Charge[/h] During use, and especially on overcharge, the water in the electrolyte splits into hydrogen and oxygen. The battery begins to gas, which results in water loss. In flooded batteries, water can be added but in sealed batteries, water loss leads to an eventual dry-out and decline in capacity. Water loss from a sealed unit can eventually cause disintegration of the separator. The initial stages of dry-out can go undetected and a drop in capacity may not be immediately evident. Early detection of this failure is important. Read about
Charging Lead Acid, under Watering.
On overcharge, a battery becomes a “water-splitting device” that turns water into oxygen and hydrogen. A parallel can be made with the
fuel cell, but this device does the opposite; it turns oxygen and hydrogen back to electricity and produces water. Turning water into hydrogen needs energy and in a battery this is in the form of overcharge. Converting hydrogen and oxygen back to water regenerates energy. Read about the
Fuel Cell.
[h=2]Acid Stratification[/h] The electrolyte of a stratified battery concentrates at the bottom, starving the upper half of the cell. Acid stratification occurs if the battery dwells at low charge (below 80 percent), never receives a full charge and has shallow discharges. Driving a car for short distances with power-robbing accessories contributes to acid stratification because the alternator cannot always apply a saturated charge. Large luxury cars are especially prone to acid stratification. This is not a battery defect per se but the result of use. Figure 1 illustrates a normal battery in which the acid is equally distributed from top to bottom.
[TABLE]
<tbody> [TR]
[TD]
[/TD]
[TD]
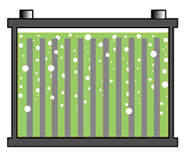
Figure 1: Normal battery
The acid is equally distributed from the top to the bottom of the battery, providing good overall performance.
Courtesy of Cadex
[/TD]
[/TR]
</tbody> [/TABLE]
Figure 2 shows a stratified battery in which the acid concentration is light on top and heavy on the bottom. The light acid on top limits plate activation, promotes corrosion and reduces the performance, while the high acid concentration on the bottom makes the battery appear more charged than it is and artificially raises the open-circuit voltage. Because of unequal charge across the plates, CCA performance, or the ability to crank the engine, is also reduced.
[TABLE]
<tbody> [TR]
[TD]
[/TD]
[TD]
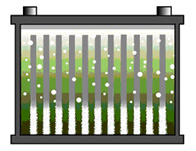
Figure 2: Stratified battery
The acid concentration is light on top and heavy on the bottom. This raises the open circuit voltage and the battery appears fully charged. Excessive acid concentration induces sulfation on the lower half of the plates.
Courtesy of Cadex
[/TD]
[/TR]
</tbody> [/TABLE]
Allowing the battery to rest for a few days, doing a shaking motion or tipping the battery on its side helps correct the problem. Applying an equalizing charge by raising the voltage of a 12-volt battery to 16 volts for one to two hours also helps by mixing the electrolyte through electrolysis. Avoid extending the topping charge beyond its recommended time.
Acid stratification cannot always be avoided. During cold winter months, starter batteries of most passenger cars dwell at a 75 percent charge level. Knowing that motor idling and driving in gridlocked traffic does not sufficiently charge the battery, a charge with an external charger may be needed from time to time. If this is not practical, switch to an AGM battery [See BU-201a, Absorbent Glass Mat (AGM)]. AGM does not suffer from acid stratification and is less subject to sulfation if undercharged than is the case with the flooded version. AGM is a little more expensive than the flooded starter battery but tends to last longer.
[h=2]Surface Charge[/h]
Lead acid batteries are sluggish and cannot convert lead sulfate to lead and lead dioxide quickly enough during charge. As a result, most of the charge activities occur on the plate surfaces. This induces a higher state-of-charge on the outside than in the inner plate. A battery with surface charge has a slightly elevated voltage. To normalize the condition, switch on electrical loads to remove about one percent of the battery’s capacity, or allow the battery to rest for a few hours. Surface charge is not a battery defect but a reversible condition resulting from charging.
[h=2]Simple Guidelines for Extending Battery Life[/h]
- Allow a fully saturated charge of 14–16 hours. Charge in a well-ventilated area.
- Always keep lead acid charged. Avoid storage below 2.10V/cell, or at a specific gravity level below 1.190.
- Avoid deep discharges. The deeper the discharge, the shorter the battery life will be. A brief charge on a 1 to 2 hour break during heavy use prolongs battery life.
- Never allow the electrolyte to drop below the tops of the plates. Exposed plates sulfate and become inactive. When low, add only enough water to cover the exposed plates before charging; fill to the correct level after charge.
- Never add acid. This would raise the specific gravity too high and cause excessive corrosion.
- Use distilled or ionized water. Tap water may be usable in some regions.
- When new, a deep-cycle battery may have a capacity of 70 percent or less. Formatting as part of field use will gradually increase performance. Apply a gentle load for the first five cycles to allow a new battery to format.
- New batteries with low capacity many not perform as well as those that begin life with a high capacity. Low performers are known to have a short life. A capacity check as part of acceptance is advisable.